When a life-saving drug disappears from pharmacy shelves, it’s rarely an accident. It’s the result of a supply chain that was too lean, too centralized, and too fragile to handle real-world shocks. The U.S. relies on foreign countries for 80% of its active pharmaceutical ingredients (APIs)-the core building blocks of medicines. China and India together produce nearly 70% of these ingredients. When geopolitical tensions rise, natural disasters strike, or a single factory shuts down, the ripple effect hits patients first. Drug shortages aren’t just inconvenient-they’re deadly. Preventing them isn’t optional. It’s a matter of public health and national security.
Why the Current System Is Broken
For decades, pharmaceutical companies chased efficiency over safety. They moved manufacturing overseas to cut costs, relying on just-in-time delivery and single-source suppliers. The math made sense on paper: lower production costs, higher profits. But it ignored one critical fact: medicine isn’t a commodity like smartphones or clothing. You can’t wait three weeks for a new shipment of insulin or antibiotics when a patient’s life depends on it. The COVID-19 pandemic exposed these flaws. When global logistics froze, hospitals ran out of sterile injectables. ICU patients couldn’t get sedatives. Cancer treatments were delayed. The FDA recorded over 300 drug shortages in 2023 alone-many tied to API supply disruptions. The problem isn’t just about quantity. It’s about quality control, regulatory delays, and lack of visibility across supplier tiers. Most companies only track their direct suppliers. But the real risks lie three, four, or even twelve steps down the chain-in a chemical plant in Shanghai, a packaging facility in Bangalore, or a logistics hub in Rotterdam.What Resilience Actually Means
Resilience in pharmaceutical supply chains isn’t about bringing everything back to the U.S. It’s about building systems that can absorb shocks without collapsing. The U.S. Department of Health and Human Services defines it clearly: the ability to anticipate, prepare for, respond to, and recover from disruptions while keeping critical drugs flowing. That means three things:- Preparedness: Knowing where your risks are before they happen.
- Response: Having plans and flexibility to keep producing during a crisis.
- Recovery: Getting back to normal faster after a disruption.
Four Practical Steps to Build Resilience
1. Map Every Tier of Your Supply Chain
Most companies only know their direct suppliers. That’s like trying to fix a car by only checking the tires. You need to see the entire engine. Leading firms now map 12 to 15 tiers of suppliers-down to the raw material vendors. Why? Because a single faulty valve from a supplier in Vietnam can halt production of a life-saving antibiotic in New Jersey. Use digital tools to track material flows. AI-powered platforms can now identify high-risk suppliers based on location, political stability, environmental factors, and past compliance records. Companies that did this saw their vulnerability detection time drop from 45 days to just 7 days.2. Dual-Source Critical Medicines
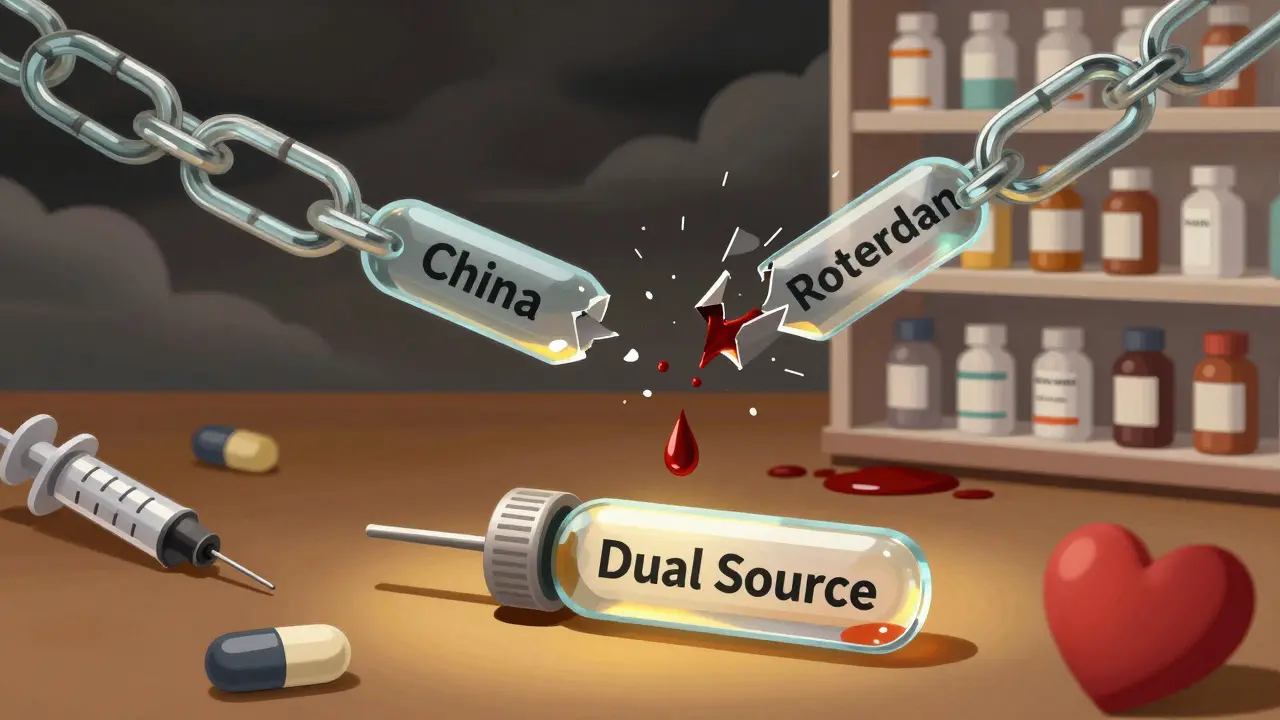
Don’t rely on one factory for one drug. Find a second supplier-even if it costs more. The goal isn’t to eliminate foreign manufacturing. It’s to avoid single points of failure. Top performers now dual-source 70-80% of their critical APIs. For example, if you get your heparin from China, find a reliable producer in Germany or Canada. It adds 5-10% to your cost of goods sold-but it prevents a $14.7 million revenue loss during a disruption, according to ZS Associates.3. Build Strategic Stockpiles
Lean inventory worked when everything moved smoothly. It doesn’t work now. For essential medicines-like epinephrine, heparin, or antibiotics-maintain 60 to 90 days of inventory. That’s not hoarding. That’s insurance. The U.S. government is stepping in too. In August 2025, an executive order created the Strategic Active Pharmaceutical Ingredients Reserve, aiming to stockpile 90-day supplies of 150 critical drugs by 2027. This isn’t just a government project. Hospitals and large pharmacy chains are starting to do the same. The cost? A few million dollars per drug. The alternative? A public health emergency.4. Invest in Modern Manufacturing
Traditional batch manufacturing is slow, wasteful, and hard to scale. Continuous manufacturing changes that. It’s like switching from a slow coffee drip to a high-speed espresso machine-constant flow, less waste, smaller footprint. Companies using continuous manufacturing see:- 30-40% smaller facilities
- 20-25% less energy use
- 15-20% less material waste
- 18-22% higher yield rates
The Real Cost of Doing Nothing
Some executives argue: “Why spend more when we’ve gotten by for years?” The answer is in the numbers. - A single major drug shortage costs large pharma firms an average of $14.7 million in lost revenue. - Companies with resilience programs see a 1.8x return on investment within three years. - The global market for supply chain resilience tools is projected to hit $9.7 billion by 2027-up from $4.2 billion in 2023. But the real cost isn’t financial. It’s human. When a child can’t get their asthma inhaler. When a cancer patient misses a chemo dose. When a senior can’t get their blood thinner. These aren’t statistics. They’re lives.What’s Holding Companies Back?
The biggest barriers aren’t technical-they’re organizational.- 78% of companies say silos between procurement, manufacturing, and regulatory teams slow down decisions.
- 65% lack integrated data systems to track suppliers across regions.
- 52% are unsure how new FDA rules will affect their operations.

What’s Next? The Future of Drug Supply Chains
By 2030, experts predict:- 35-40% of U.S. pharmaceuticals will be made domestically (up from 28% today).
- 65-70% of supply will come from regional networks across North America, Europe, and Asia-not just one country.
- 45-50% of new manufacturing capacity will use continuous processes.
Final Thought: Resilience Isn’t an Expense. It’s an Investment.
Building a resilient pharmaceutical supply chain doesn’t mean going back to the past. It means building a smarter future-one that values reliability over cheapness, safety over speed, and people over profit margins. The technology exists. The data is clear. The policy is shifting. What’s missing is the will to act before the next crisis hits.Why are drug shortages still happening in 2025?
Drug shortages persist because supply chains are still too centralized and overly reliant on a few foreign manufacturers for active pharmaceutical ingredients (APIs). Even with new regulations and investments, many companies haven’t fully mapped their supplier networks or diversified sourcing. A single factory shutdown, export ban, or quality control failure can ripple across the entire system. The gap between policy intent and operational execution remains wide.
Can the U.S. make all its own drugs?
No, and trying to do so would be impractical and expensive. The U.S. currently produces only 28% of essential medicine APIs domestically. Rebuilding full domestic capacity would require over $120 billion in global investment and take decades. More importantly, it would create new risks-like over-reliance on a single domestic supplier. The goal isn’t self-sufficiency. It’s resilience through balance: strategic domestic capacity for critical drugs, combined with diversified global sourcing.
How much does it cost to build a resilient supply chain?
It varies by company size. Large pharmaceutical firms spend 5-10% of their annual supply chain budget on resilience. For a $5 billion company, that’s $250-500 million over five years. Costs include dual sourcing, buffer stock, digital tools, and modern manufacturing upgrades. But the ROI is clear: companies avoid an average of $14.7 million in losses per major disruption. The cost of inaction is far higher.
What’s the role of AI in preventing drug shortages?
AI helps predict disruptions before they happen. By analyzing global events-weather patterns, political unrest, shipping delays, and supplier compliance history-AI models can forecast risks with 85-90% accuracy up to 90 days in advance. This gives companies time to reroute shipments, activate backup suppliers, or ramp up production. Companies using AI for risk forecasting have reduced response times by 40-60% during crises.
Are generic drug makers more vulnerable to shortages?
Yes. Generic drug manufacturers operate on razor-thin margins, so they’re less likely to invest in buffer stock, dual sourcing, or advanced manufacturing. Over 80% of U.S. generic drugs are made overseas, often in just one facility. When that facility fails, the entire market feels it. That’s why the Strategic Active Pharmaceutical Ingredients Reserve focuses heavily on generics-it’s where the biggest gaps are.
How can hospitals prepare for drug shortages?
Hospitals should maintain 60-90 days of inventory for critical drugs like antibiotics, sedatives, and IV fluids. They should also join regional supply-sharing networks and use real-time shortage alerts from the FDA and ASHP. Many hospitals now have a dedicated supply chain risk officer who works with pharmacy teams to identify alternatives and prioritize usage during shortages. Proactive planning saves lives.


Comments
Been in pharma logistics for 15 years. This post nails it. We’re not talking about fancy gadgets here - it’s about having a backup plan when your only supplier in Shanghai goes dark. Simple stuff, really.
ALERT: Big Pharma + China = controlled demolition of your medicine cabinet. 🚨 They’re poisoning us AND holding our insulin hostage. The FDA’s asleep at the wheel. #WakeUpAmerica 🇺🇸💊
Let me get this straight - we’ve outsourced the literal building blocks of human survival to countries that don’t give a damn about our patients? And you call that capitalism? This isn’t efficiency. It’s corporate suicide with a PowerPoint deck. 🤡
ok so i read this and i’m like… wait… so if we just… like… make more stuff here…? but then the cost… and also i saw a tweet that said the chinese government owns 60% of the api factories?? is that true??
Y’all acting like this is news. I’ve been screaming about this since 2020. The system’s a house of cards made of cheap labor and wishful thinking. And now? We’re all just waiting for the next sneeze to knock it over. 🤦♀️
Canada’s been doing this right for years. We don’t outsource our insulin. We don’t gamble with antibiotics. Why? Because we value human life over quarterly earnings. America’s got a leadership problem - and it’s not in DC.
Mapping supplier tiers? That’s not optional. That’s basic hygiene. If you don’t know where your raw materials come from, you’re not a manufacturer - you’re a middleman with a fancy logo.
People are dying because CEOs are too lazy to spend an extra 5% on safety. And you wonder why trust in institutions is gone? It’s because we let greed win - again.
My aunt missed her chemo because the heparin ran out. They gave her saline instead. She’s fine now. But what if she wasn’t? This isn’t a ‘supply chain issue.’ It’s a moral failure.
why is everyone so scared of china? we import phones laptops clothes food why not medicine? its just another product. you all are overreacting
Just a note: The CHIPS Act funding extension includes API infrastructure. It’s not just talk. There’s real capital moving. We’re seeing pilot plants in Ohio and Kentucky already.
Let me tell you something - I work in a hospital pharmacy in Delhi, and we’ve been living this reality for years. When a drug disappears, we don’t wait for the FDA to fix it. We call our contacts in Bangladesh, Pakistan, even Kenya. We make it work. The U.S. has the tech, the money, the brains - but it’s stuck in boardroom paralysis. We don’t need more reports. We need action. And we need to stop treating medicine like a stock option.
Continuous manufacturing is the future. I’ve seen it. It’s not sci-fi - it’s happening in North Carolina right now. Smaller footprint, less waste, faster turnaround. Why aren’t we building ten of these a year? Because old habits die hard. And someone’s making bank off the chaos.
So we’re supposed to trust the same corporations that cut corners for 20 years to suddenly become saints? Nah. Policy changes are great. But without transparency, accountability, and real penalties for negligence? It’s just theater.
Does anyone track how many deaths are directly tied to drug shortages? Not ‘estimated’ - actual death certificates? I think we’re undercounting.
Yeah, and that’s why hospitals are starting to do their own stockpiling now. One in three has a dedicated buffer stock for top 10 critical drugs. It’s not perfect, but it’s a start.
Exactly. And the FDA’s new guidance on AI-driven risk modeling? It’s a game-changer. We’re moving from reactive to predictive. That’s the real win.
And the best part? The ROI timeline is shrinking. Last year, it was 5 years. Now? 2.5 years for most mid-sized firms. That’s what gets execs to move.
So we’re finally admitting that profit isn’t a substitute for survival? Took long enough.
Meanwhile, my cousin’s kid still can’t get his asthma inhaler. So yeah - keep talking. We’ll be here.