Biologic drugs can cost thousands of dollars a month. For conditions like rheumatoid arthritis, Crohn’s disease, or certain cancers, patients often face bills of $6,000 to $10,000 per dose. That’s not a typo. But there’s a growing alternative: biosimilars. These aren’t cheap knockoffs. They’re scientifically approved copies of complex biologic medicines-and they’re already cutting costs for millions. Understanding them isn’t just about saving money. It’s about knowing if they’re right for you.
What Exactly Are Biosimilars?
Biosimilars are not like generic pills. You can’t just swap one for another like you would with aspirin or metformin. Biologics are made from living cells-yeast, bacteria, or animal cells. That means each batch has tiny natural variations. Even the original drug, called a reference product, isn’t perfectly identical from one vial to the next. Biosimilars have to match that level of complexity. The U.S. Food and Drug Administration (FDA) says a biosimilar must be highly similar to the reference biologic, with no clinically meaningful differences in safety, purity, or potency. That means the same mechanism of action, same dosing, same side effects, same effectiveness. The FDA doesn’t require new large-scale trials for every single condition. Instead, they use a total evidence approach: comparing molecular structure, lab tests, animal studies, and small human trials. If everything lines up, the biosimilar gets approved. The first one approved in the U.S. was Zarxio in 2015, a copy of filgrastim used to boost white blood cells after chemotherapy. Since then, the FDA has approved 45 biosimilars as of October 2023. They’re used for cancer, autoimmune diseases like psoriasis and lupus, and inflammatory bowel disease.How Much Do Biosimilars Actually Save?
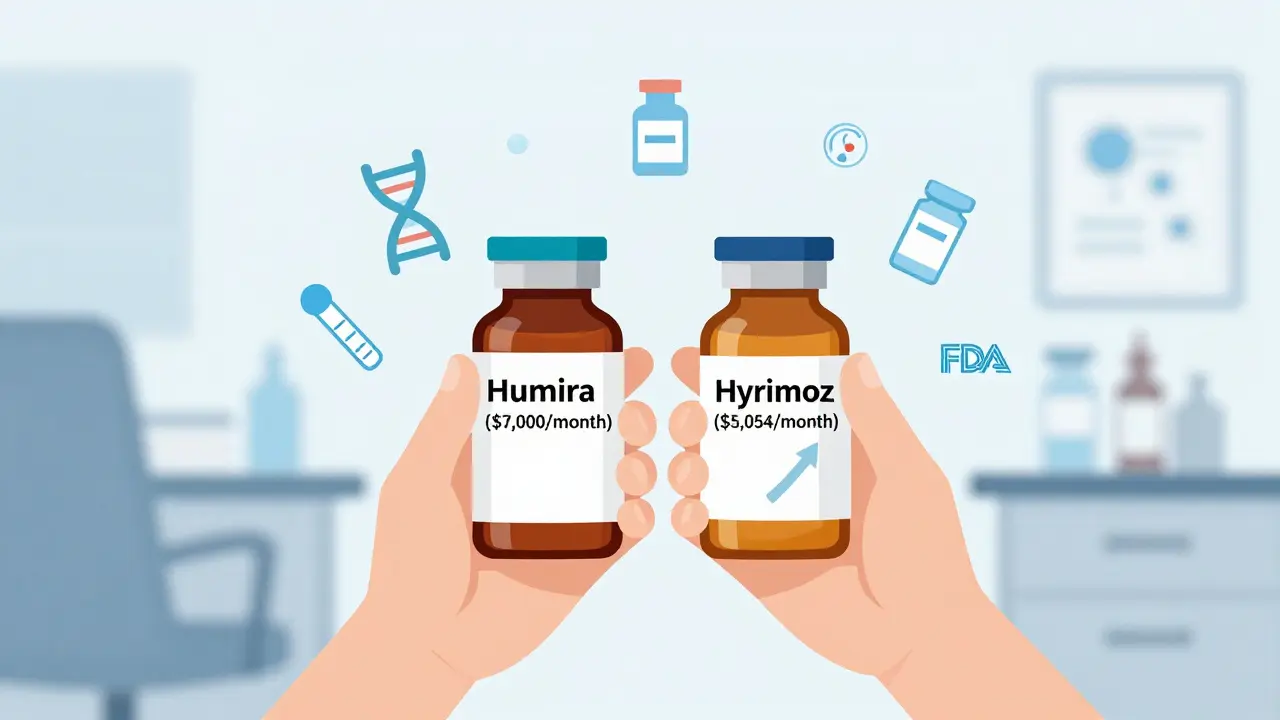
Here’s where things get practical. Generic small-molecule drugs often cut prices by 80% or more. Biosimilars? They start at 15% to 30% cheaper. That might sound underwhelming. But when the original drug costs $7,000 a month, even 20% off is $1,400 saved per patient every 30 days. Take Humira, the world’s best-selling biologic. Before biosimilars hit the U.S. market in 2023, it cost about $7,000 per month. The first interchangeable biosimilar, Hyrimoz, launched at $5,054-a 28% drop. Other Humira biosimilars followed, some priced even lower. That’s not just a discount. That’s life-changing for patients with high-deductible plans or no insurance. The savings add up fast. The Congressional Budget Office estimated biosimilars could save the U.S. healthcare system $150 billion by 2030. The RAND Corporation projected $54 billion in savings between 2017 and 2026. But real-world savings are slower than expected. Why? Because of how the system works. Pharmacy benefit managers, insurers, and drug manufacturers often lock in deals that keep prices high. Sometimes, the list price drops, but your copay stays the same. A 2022 patient survey by the Arthritis Foundation found 72% of people using biosimilars reported lower out-of-pocket costs. But 28% still worried about effectiveness-until they talked to their doctor.Biosimilars vs. Reference Products: Are They Really the Same?
The biggest fear patients have: “Will this work as well?” The answer, backed by science, is yes. The NOR-SWITCH trial, published in The Lancet in 2016, followed over 500 patients with autoimmune diseases who switched from a reference biologic to its biosimilar. After 52 weeks, there was no increase in disease flare-ups, side effects, or immune reactions. Similar results have been seen in dozens of other studies across cancer, arthritis, and IBD. Dr. Gary Lyman, a cancer specialist at Fred Hutchinson Cancer Research Center, put it plainly in a 2022 JAMA Oncology editorial: “Biosimilars have demonstrated equivalent efficacy and safety profiles compared to their reference products across multiple clinical trials involving thousands of patients.” The European Medicines Agency (EMA) has been approving biosimilars since 2006. Over 16 years of real-world use, they’ve seen no unexpected safety issues. The FDA says the same: “Biosimilars are as safe and effective as their reference products.” But there’s a catch. Some patients are nervous about switching. And that’s valid. If you’ve been stable on a drug for years, changing to a new one-even a scientifically identical one-can feel risky. That’s why many doctors take time to explain the data. One rheumatologist on Reddit said he spends 10 to 15 minutes with each patient just to walk through the evidence.
Interchangeable vs. Biosimilar: What’s the Difference?
Not all biosimilars are created equal in terms of substitution. The FDA has two categories: biosimilar and interchangeable. A biosimilar can be prescribed by a doctor and filled by a pharmacist, just like the original. But a pharmacist can’t automatically swap it in without the prescriber’s okay-unless it’s designated as “interchangeable.” An interchangeable biosimilar has passed extra testing to prove that switching back and forth between it and the reference product won’t increase risk or reduce effectiveness. As of November 2023, only six biosimilars have that status in the U.S. Hyrimoz, the Humira copy, was the first in July 2023. This matters because in 48 states, pharmacists can substitute an interchangeable biosimilar without telling the doctor. In the other two states, they still need permission. This rule is meant to protect patients from unintended switches. But it also slows down adoption.Why Aren’t Biosimilars Used More Often?
You’d think with proven safety and lower prices, biosimilars would be everywhere. But adoption varies wildly. For infliximab (Remicade), biosimilars grabbed 72% of new prescriptions within 18 months of launch. But for etanercept (Enbrel), only 28% took the biosimilar version. Why the gap? Patents. Drugmakers use legal tactics called “product hopping”-making small changes to the original drug and filing new patents to block biosimilars. They also offer deep discounts to insurers to lock them into exclusive deals. Sometimes, a biosimilar is cheaper, but the insurer won’t cover it unless you try the brand-name drug first. Another issue? Provider knowledge. A 2022 survey by the American Society of Clinical Oncology found 78% of oncologists needed 1 to 2 hours of training to feel confident prescribing biosimilars. The biggest barrier? “Navigating payer-specific coverage requirements.” Patients aren’t always informed either. Many don’t know they’re being prescribed a biosimilar until they see a lower bill-or a different name on the label.What Does This Mean for You?
If you’re on a biologic drug and paying high out-of-pocket costs, ask your doctor: “Is there a biosimilar available for my medication?” Check the FDA’s Purple Book-it’s the official list of approved biosimilars and their reference products. It’s free and updated regularly. If your doctor suggests switching, ask:- Is this biosimilar approved as interchangeable?
- Will my insurance cover it at a lower cost?
- Has this been studied in patients like me?
- What happens if I have a reaction?
What’s Next for Biosimilars?
The next wave is coming fast. High-revenue biologics like Stelara (ustekinumab), Dupixent (dupilumab), and Enbrel are losing patent protection in the next few years. Seven biosimilar applications for Stelara alone are pending FDA review as of September 2023. The Inflation Reduction Act of 2022 is helping too. Starting in 2024, Medicare Part D patients will pay only 25% out-of-pocket for biosimilars-down from 50% or more. That’s a huge incentive for both patients and insurers. Some experts predict biosimilar use will jump from 25-30% to 50-60% of eligible prescriptions by 2027. That could mean billions in savings for patients and the system. But it won’t happen unless patients speak up. Ask questions. Demand transparency. If your pharmacy gives you a new bottle and you don’t recognize the name, call your doctor. You’re not being difficult-you’re being informed.Frequently Asked Questions
Are biosimilars safe?
Yes. The FDA requires extensive testing to prove biosimilars are as safe and effective as the original biologic. Over 16 years of use in Europe and nearly a decade in the U.S. have shown no unexpected safety issues. Large studies, including the NOR-SWITCH trial, found no increased risk of side effects or loss of effectiveness when switching from a reference product to a biosimilar.
Why are biosimilars cheaper if they’re just as good?
Biosimilars don’t need to repeat expensive, large-scale clinical trials because they’re built on the existing data from the original drug. The approval process is shorter and less costly. That savings gets passed on-but not always directly to patients. Drug pricing systems, insurance contracts, and manufacturer rebates can slow down how much you actually save at the pharmacy.
Can I switch from my biologic to a biosimilar on my own?
No. Always talk to your doctor before switching. Even if a biosimilar is labeled as “interchangeable,” your provider should be involved. They know your medical history and can monitor for any changes. Some states allow pharmacists to substitute interchangeable biosimilars without a new prescription-but even then, it’s best to be informed.
Do biosimilars cause more side effects or immune reactions?
No. Studies show no higher rate of immune reactions with biosimilars compared to the original biologics. The manufacturing process ensures that any minor differences in structure don’t affect how the drug interacts with your immune system. The FDA requires specific immunogenicity testing as part of approval.
How do I know if I’m getting a biosimilar?
Check the drug name. Biosimilars have a four-letter suffix added to the generic name (like adalimumab-adaz for Hyrimoz). Your pharmacy label will list the full name. If you’re unsure, ask your pharmacist or call your doctor’s office. You can also look up your drug in the FDA’s Purple Book to see if a biosimilar version exists.
Will my insurance cover a biosimilar?
Most do, especially since the Inflation Reduction Act lowered out-of-pocket costs for Medicare Part D users starting in 2024. But coverage rules vary. Some insurers require you to try the brand-name drug first (called step therapy). Others may only cover certain biosimilars. Always check with your plan before switching.
Next Steps
- Look up your current biologic drug in the FDA Purple Book to see if a biosimilar exists.
- Ask your doctor: “Is there a biosimilar option for my medication?”
- Call your insurance company and ask: “What’s my out-of-pocket cost for the biosimilar vs. the brand-name drug?”
- If you’re switching, keep track of how you feel for the first few weeks. Report any changes to your provider.
Biosimilars aren’t magic. They’re science-carefully tested, closely monitored, and proven to work. And they’re one of the clearest paths we have to making life-saving drugs affordable. The question isn’t whether they’re safe. It’s whether you’ll let cost stop you from asking for them.


Comments
I was on Humira for 5 years. Switched to Hyrimoz last year. My bill dropped from $800 to $120 a month. No flare-ups. No weird side effects. Honestly? I’m just glad I didn’t die trying to afford my medicine.
The system is rigged. Biosimilars save billions but patients still get stuck with high copays because PBMs and pharma cut secret deals. The FDA says they’re safe, but the real enemy is the insurance middlemen who profit from confusion.
Biosimilars are just corporate lies wrapped in FDA stickers. They’re cheaper because they’re inferior. I read a guy in Australia who got a rash after switching. You think that’s coincidence? Nah. It’s profit over people.
It is imperative that patients be fully informed regarding the distinction between biosimilars and reference products. Misunderstanding this can lead to non-adherence and potential clinical deterioration. The onus is on healthcare providers to ensure comprehensive education prior to any transition.
I switched to a biosimilar and my husband said I looked "different"... like I was fading. I think the drug messed with my hormones. I had nightmares for two weeks. My mom said it’s because I’m too sensitive. But what if it’s the biosimilar? What if they’re poisoning us slowly?
Just switched to a biosimilar for my psoriasis 😊 My out-of-pocket dropped from $750 to $90. I’m not crying, you’re crying. This is what healthcare should look like. Thank you science 🙏
The NOR-SWITCH trial is solid but most docs don’t even know what’s approved. I asked mine about a Stelara biosimilar and he said "I’ve never heard of that". We’re talking about a $10B drug and he’s clueless
I get why people are scared to switch. I was too. But my rheumatologist sat with me for 20 minutes, showed me the studies, and said "you’re not taking a gamble, you’re taking a smarter option." It helped.
This whole biosimilar thing is just Big Pharma’s way of tricking people into taking inferior drugs so they can charge more for the next version. I saw a documentary. They’re already planning the next patent cliff.
I got my biosimilar and now my insurance says I have to switch every 6 months. Like I’m a robot. They swap me between 3 different ones. I’m scared to even take them. What if one day I just... stop working?
If you’re considering a switch, talk to your pharmacist. They know the copay differences better than your doctor sometimes. And don’t be afraid to ask for the Purple Book link. You’re not being difficult-you’re being smart.
You think this is about savings? It’s about control. The pharma companies don’t want you to know you can get the same drug for less. They want you dependent. And they’re winning.